Are you scouring the internet for 'how to write formulas of binary ionic compounds'? Here you can find questions and answers on this topic.
Table of contents
- How to write formulas of binary ionic compounds in 2021
- Writing formulas for binary ionic compounds worksheet answers
- Writing chemical formulas for binary ionic compounds worksheet
- Writing formulas for ionic compounds answers
- Ionic binary compound rules
- Rules for naming ionic compounds
- Binary ionic compound formula calculator
- Naming ionic compounds
How to write formulas of binary ionic compounds in 2021
 This picture shows how to write formulas of binary ionic compounds.
This picture shows how to write formulas of binary ionic compounds.
Writing formulas for binary ionic compounds worksheet answers
 This picture representes Writing formulas for binary ionic compounds worksheet answers.
This picture representes Writing formulas for binary ionic compounds worksheet answers.
Writing chemical formulas for binary ionic compounds worksheet
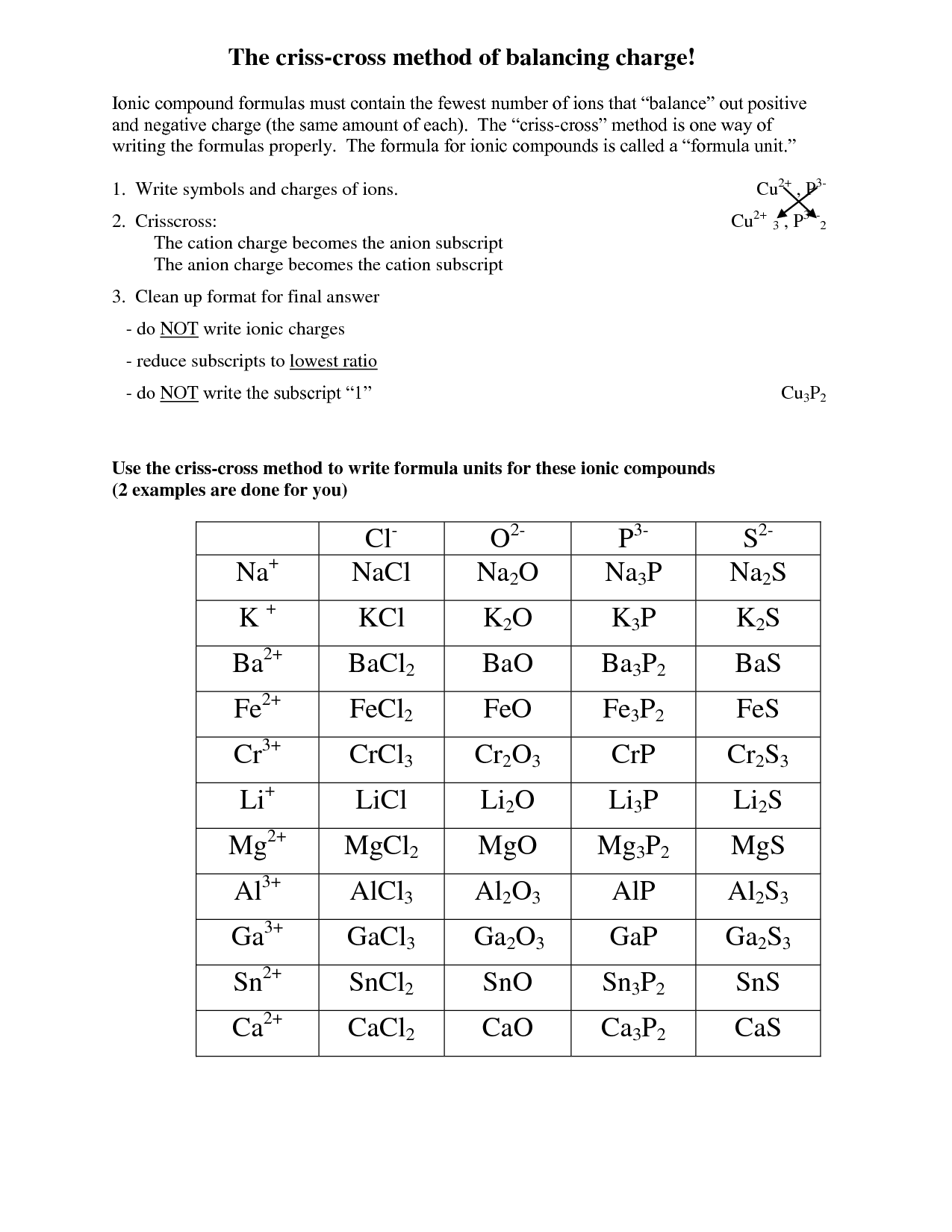 This picture illustrates Writing chemical formulas for binary ionic compounds worksheet.
This picture illustrates Writing chemical formulas for binary ionic compounds worksheet.
Writing formulas for ionic compounds answers
 This image representes Writing formulas for ionic compounds answers.
This image representes Writing formulas for ionic compounds answers.
Ionic binary compound rules
.PNG) This picture illustrates Ionic binary compound rules.
This picture illustrates Ionic binary compound rules.
Rules for naming ionic compounds
.PNG) This image demonstrates Rules for naming ionic compounds.
This image demonstrates Rules for naming ionic compounds.
Binary ionic compound formula calculator
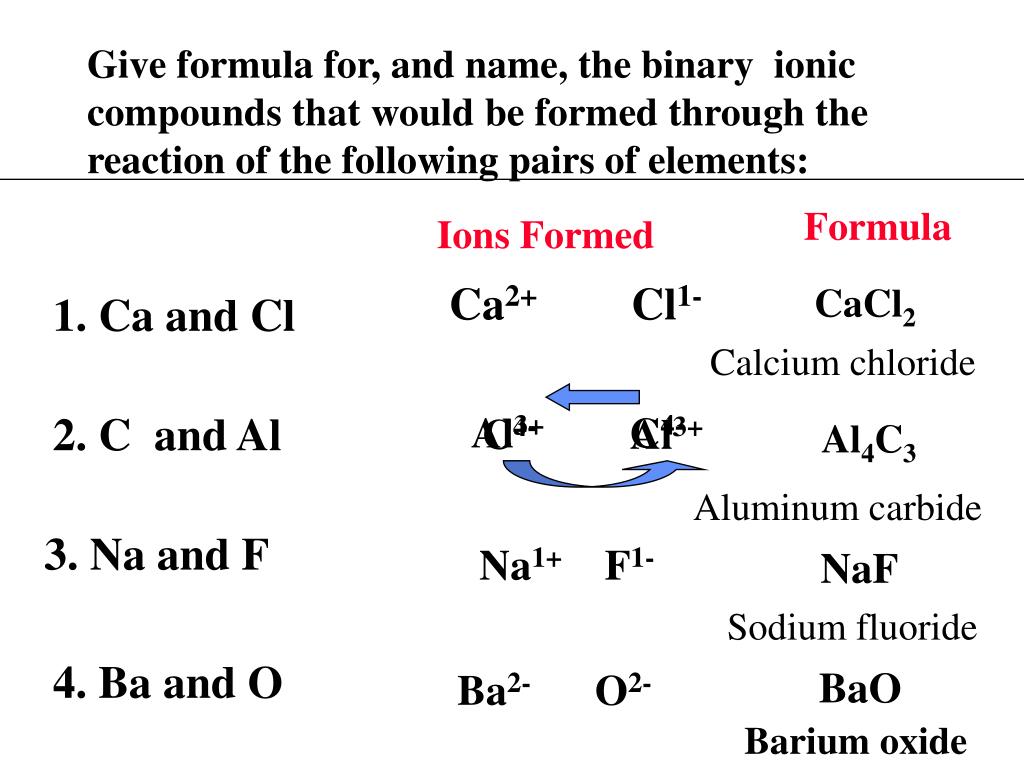 This image shows Binary ionic compound formula calculator.
This image shows Binary ionic compound formula calculator.
Naming ionic compounds
.PNG) This picture representes Naming ionic compounds.
This picture representes Naming ionic compounds.
Why does an iron ion have a 2 + charge?
In the first compound, the iron ion has a 2+ charge because there are two Cl − ions in the formula (1− charge on each chloride ion). In the second compound, the iron ion has a 3+ charge, as indicated by the three Cl − ions in the formula.
How to write a formula for an ionic compound?
An alternative way to writing a correct formula for an ionic compound is to use the crisscross method. In this method, the numerical value of each of the ion charges is crossed over to become the subscript of the other ion. Signs of the charges are dropped. Write the formula for lead (IV) oxide.
How are ionic compounds named in Roman numerals?
Some metals, primarily, but not limited to, the transition metals can form more than one positively charged ion, such as Fe2+ and Fe3+, the ion is named for the charge using Roman numerals, such as iron (II) and iron (III). Binary ionic compounds are named by writing the metal ion first, followed by the nonmetal.
How do you name a binary ionic compound?
Binary ionic compounds are named by writing the metal ion first, followed by the nonmetal. If the metal has only one ion, then the Roman numeral is not necessary.
Last Update: Oct 2021
Leave a reply
Comments
Shealynn
23.10.2021 07:47The names are saved by finding the intersection between the cations and anions. Worksheet writing binary formulas answers 66.
Kimm
24.10.2021 00:45Composition formulas of geographic region compounds. When you indite the formulas for binary compounds, they will consist of two elemental symbols, and they May also have 1 or two inferior numbers, if the elements don't combining in a 1 to one ratio.
Sheriee
21.10.2021 04:59Appellative compounds - partly 2 - youtube: this video explains how to usance a chemical epithet to write the formula for that compound. This worksheet has 20 problems to solve.