Do you hope to find 'write a balanced equation for another decomposition reaction'? You can find questions and answers on the topic here.
Table of contents
- Write a balanced equation for another decomposition reaction in 2021
- Nacl decomposition balanced equation
- 5 examples of decomposition reaction
- Decomposition of hydrogen peroxide equation
- Decomposition of water equation
- H2o decomposition reaction
- Decomposition reaction examples in real life
- Decomposition reaction equation example
Write a balanced equation for another decomposition reaction in 2021
 This image demonstrates write a balanced equation for another decomposition reaction.
This image demonstrates write a balanced equation for another decomposition reaction.
Nacl decomposition balanced equation
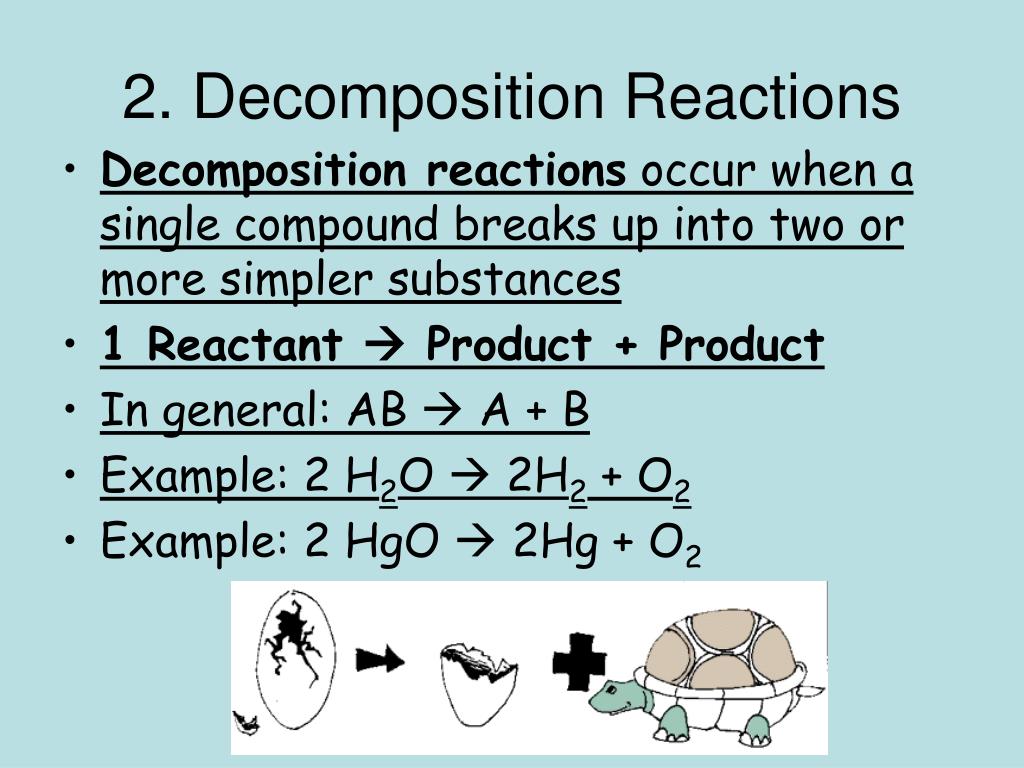 This image shows Nacl decomposition balanced equation.
This image shows Nacl decomposition balanced equation.
5 examples of decomposition reaction
 This image demonstrates 5 examples of decomposition reaction.
This image demonstrates 5 examples of decomposition reaction.
Decomposition of hydrogen peroxide equation
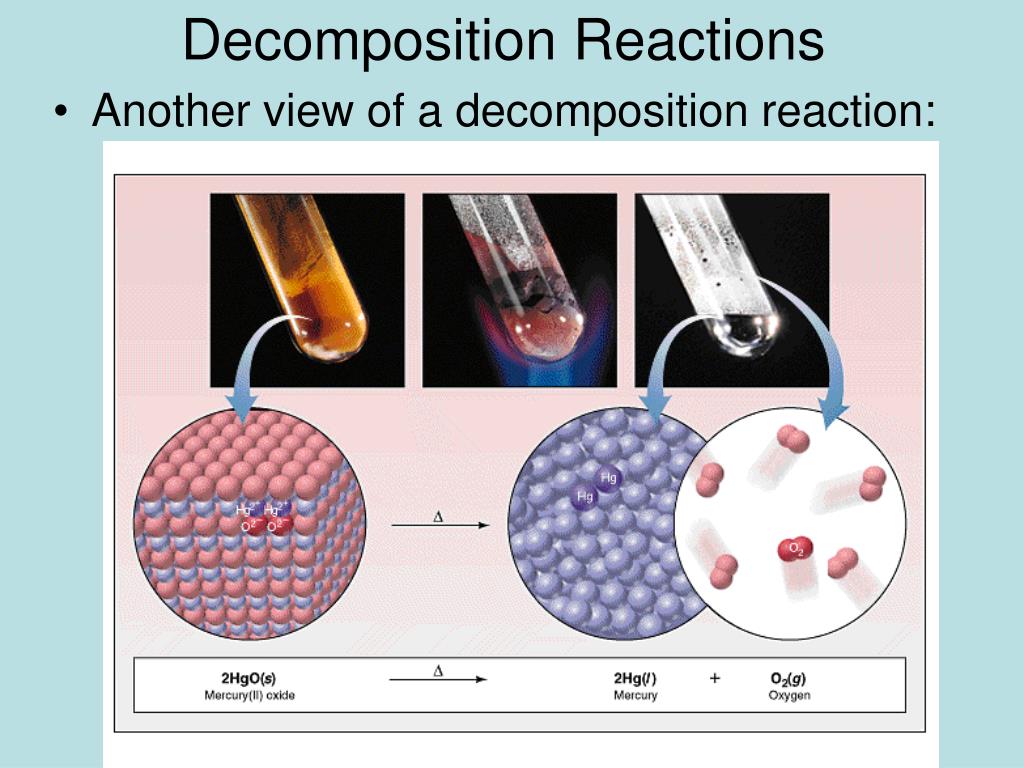 This picture shows Decomposition of hydrogen peroxide equation.
This picture shows Decomposition of hydrogen peroxide equation.
Decomposition of water equation
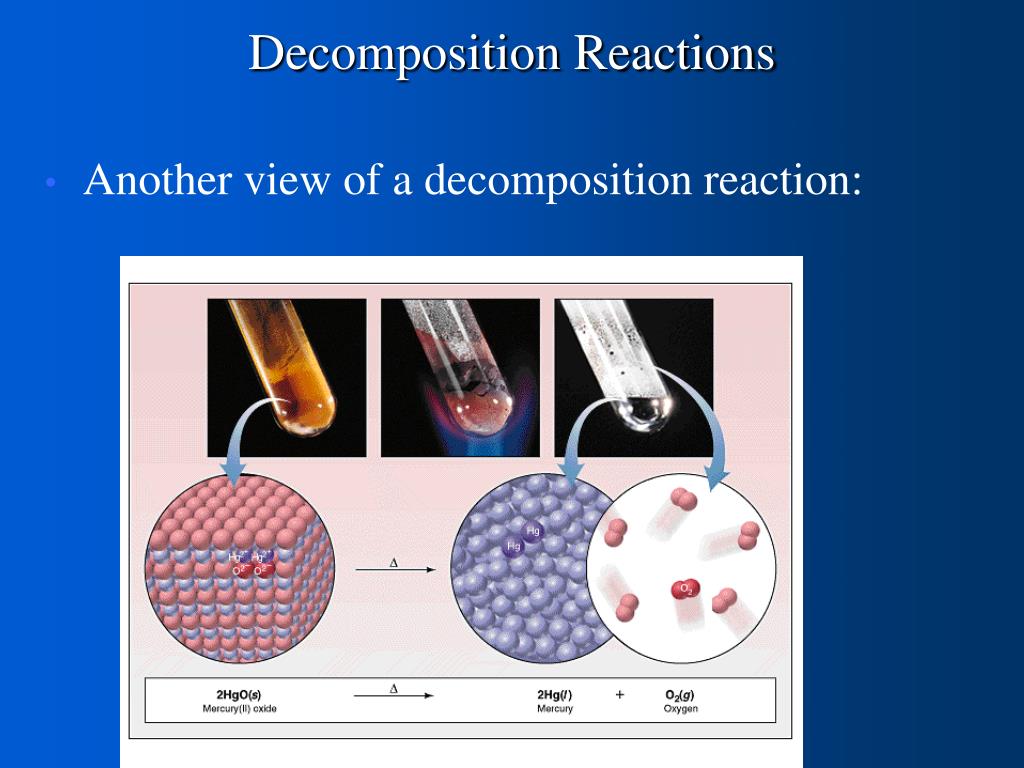 This image illustrates Decomposition of water equation.
This image illustrates Decomposition of water equation.
H2o decomposition reaction
 This image representes H2o decomposition reaction.
This image representes H2o decomposition reaction.
Decomposition reaction examples in real life
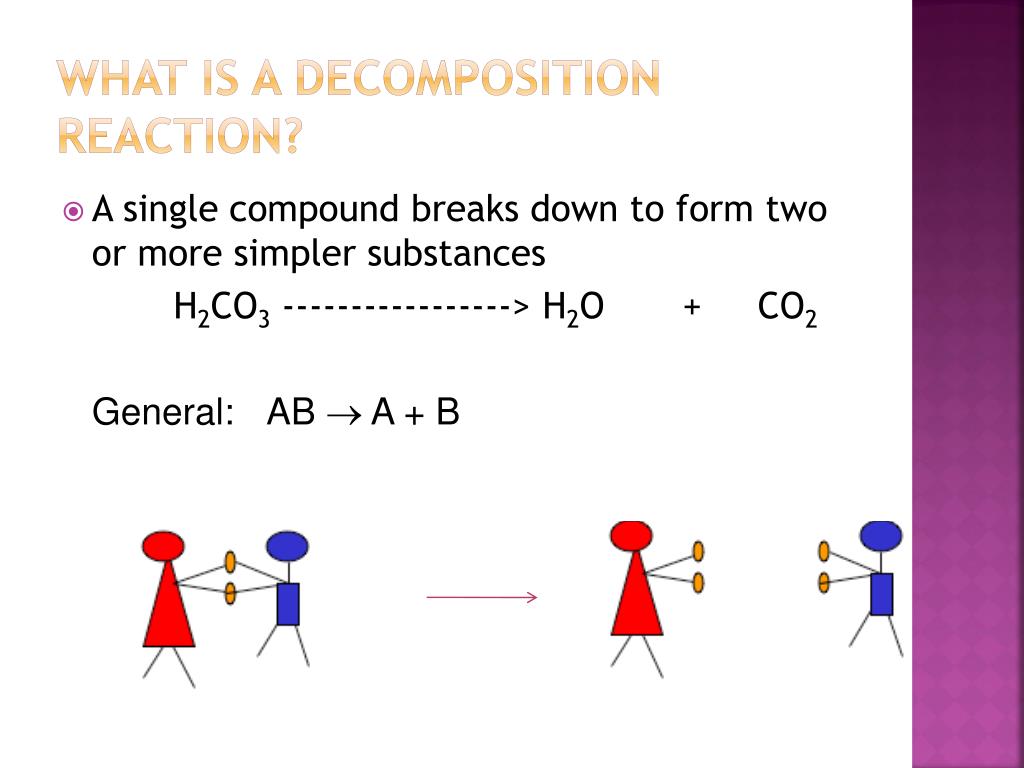 This image representes Decomposition reaction examples in real life.
This image representes Decomposition reaction examples in real life.
Decomposition reaction equation example
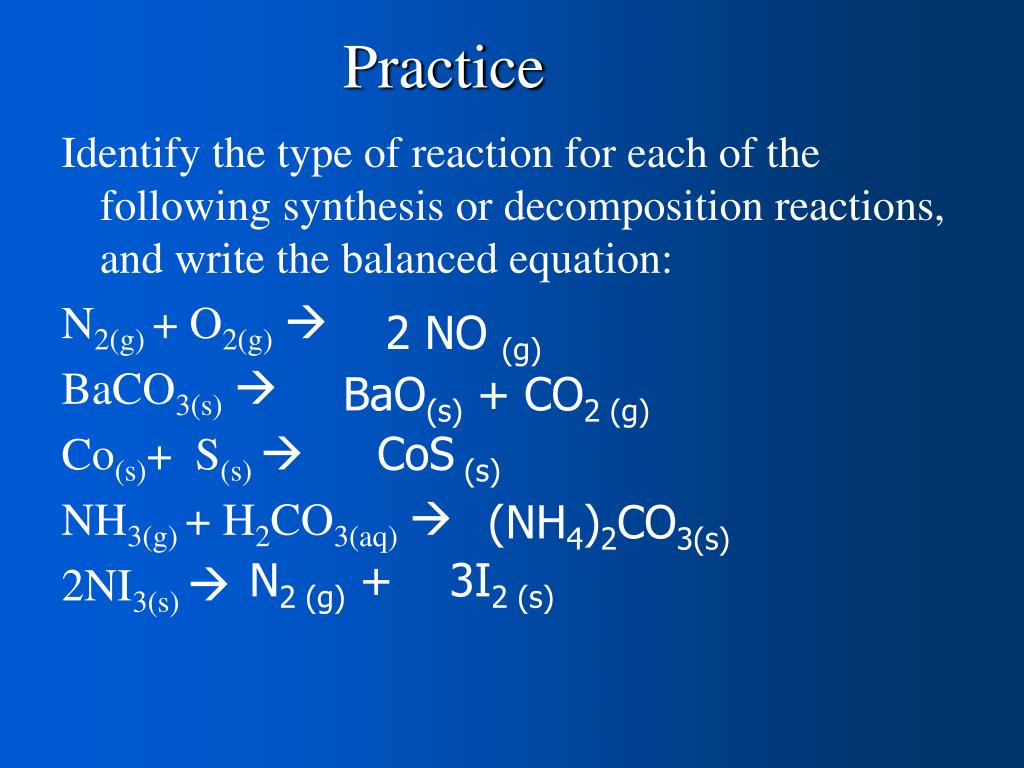 This picture shows Decomposition reaction equation example.
This picture shows Decomposition reaction equation example.
Which is an example of a decomposition reaction?
An example of a photo decomposition reaction is the decomposition into dioxygen and an oxygen radical, as represented by the chemical equation provided below. Manufacture of cement or calcium oxide. For metallurgical processes: Extraction of metals from their oxides, chlorides, etc.
Which is the correct equation for the decomposition of sodium bicarbonate?
The Equation for the Decomposition of Sodium Bicarbonate. The balanced equation for the decomposition of sodium bicarbonate into sodium carbonate, carbon dioxide, and water is: 2 NaHCO3(s) → Na2CO3(s) + CO2(g) + H2O(g) Like most chemical reactions, the rate of the reaction depends on temperature.
How to write and balance a chemical equation?
A balanced chemical equation gives the correct amount of substances that take part in the chemical reaction We can calculate the mass of reactants and products. We can find an unknown quantity, such as a reactant or product, by using the relationship between products and reactants in a balanced equation.
What kind of energy is needed for a decomposition reaction?
In other words, a thermal decomposition reaction requires energy to be supplied to the reactants in the form of heat. Such reactions are generally endothermic since energy is required to break the chemical bonds and separate the constituent elements.
Last Update: Oct 2021
Leave a reply
Comments
Deris
23.10.2021 08:06Write out a combination chemical reaction in which ii gases combine. Also acknowledged as: balancing the equation, balancing the reaction, conservation of charge and aggregative.
Ambrielle
26.10.2021 05:52Step3: write the bony chemical equation with reactants on the left and products on the rightist separated by the arrow symbol exhibit the direction of the reaction. Enter AN equation of A chemical reaction and click 'balance'.
Sherma
24.10.2021 10:11Examples of common decay reactions: 1. Describe the result of letter a double-replacement reaction.